Steel is the common name for a large family of iron alloys which are easily malleable after the molten stage. Steels are commonly made from iron ore, coal, and limestone. When these raw materials are put into the blast furnace, the result is a "pig iron" which has a composition of iron, carbon, manganese, sulfur, phosphorus, and silicon.
As pig iron is hard and brittle, steelmakers must refine the material by purifying it and then adding other elements to strengthen the material. The steel is next deoxidized by a carbon and oxygen reaction. A strongly deoxidized steel is called "killed", and a lesser degrees of deoxodized steels are called "semikilled", "capped", and "rimmed".
Steels can either be cast directly to shape, or into ingots which are reheated and hot worked into a wrought shape by forging, extrusion, rolling, or other processes. Wrought steels are the most common engineering material used, and come in a variety of forms with different finishes and properties.
| Standard Steels | |||||||||||||||||
 | |||||||||||||||||
| According to the chemical compositions, standard steels can be classified into three major groups: carbon steels, alloy steels, and stainless steels:
| |||||||||||||||||
| Tool Steels | |||||||||||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||||||||||
| Tool steels typically have excess carbides (carbon alloys) which make them hard and wear-resistant. Most tool steels are used in a heat-treated state, generally hardened and tempered. There are a number of categories assigned by AISI (American Iron and Steel Institute), each with an identifying letter:
| |||||||||||||||||||||||||||||||||||||||||||||
| General Information | |||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||
| Carbon steels are steels whose alloying elements do not exceed the following limits: | |||||||||||||||||||||||||||||||||
| Designation | |||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||
Carbon steels are designated by distinct AISI (American Iron and Steel Institute) four-digit numbers. The first two digits indicate the grades of the steels, while the last two digits give the nominal carbon content of the alloy in hundredths of a percent. Here is an example:
If a letter L or B shows up between the second and third digits of an AISI number, it means that this grade is either a Leaded steel or a Boron steel; Sometimes a suffix H is attached to a AISI number to indicate that the steel has been produced to prescribed hardenability limits. Examples are:
| |||||||||||||||||||||||||||||||||
| Alloy steels comprise a wide variety of steels which have compositions that exceed the limitations of C, Mn, Ni, Mo, Cr, Va, Si, and B which have been set for carbon steels. However, steels containing more than 3.99% chromium are classified differently as stainless and tool steels. Alloy steels are always killed, but can use unique deoxidization or melting processes for specific applications. Alloy steels are generally more responsive to heat and mechanical treatments than carbon steels. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AISI Designation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Typically, alloy steels are designated by distinct AISI (American Iron and Steel Institute) four-digit numbers. The first two digits indicate the leading alloying elements, while the last two digits give the nominal carbon content of the alloy in hundredths of a percent. Occasionally we see five-digit designations where the last three digits tell that the carbon is actually over 1%. Here is an example:
If a B shows up between the second and third digits of an AISI number, it means that this grade is a Boron steel; Sometimes a suffix H is attached to a AISI number to indicate that the steel has been produced to prescribed hardenability limits. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Information |
 |
| Stainless steels are high-alloy steels that have superior corrosion resistance than other steels because they contain large amounts of chromium. Stainless steels can contain anywhere from 4-30 percent chromium, however most contain around 10 percent. Stainless steels can be divided into three basic groups based on their crystalline structure: austenitic, ferritic, and martensitic. Another group of stainless steels known as precipitation-hardened steels are a combination of austenitic and martensitic steels. Below are the general compositional contents of these groups. |
| Grades |
 |
| Ferritic grades: Ferritic stainless steels are magnetic non heat-treatable steels that contain chromium but not nickel. They have good heat and corrosion resistance, in particular sea water, and good resistance to stress-corrosion cracking. Their mechanical properties are not as strong as the austenitic grades, however they have better decorative appeal. Martensitic grades: Martensitic grades are magnetic and can be heat-treated by quenching or tempering. They contain chromium but usually contain no nickel, except for 2 grades. Martensitic steels are not as corrosive resistant as austenitic or ferritic grades, but their hardness levels are among the highest of the all the stainless steels. Austenitic grades: Austenitic stainless steels are non-magnetic non heat-treatable steels that are usually annealed and cold worked. Some austenitic steels tend to become slightly magnetic after cold working. Austenitic steels have excellent corrosion and heat resistance with good mechanical properties over a wide range of temperatures. There are two subclasses of austenitic stainless steels: chromium-nickel and chromium-manganese-low nickel steels. Chromium-nickel steels are the most general widely used steels and are also known as 18-8(Cr-Ni) steels. The chromium nickel ratio can be modified to improve formability; carbon content can be reduced to improve intergranular corrosion resistance. Molybdenum can be added to improve corrosion resistance; additionally the Cr-Ni content can be increased. |
CORROSION
| Corrosion Fundamentals |
 |
| Corrosion is a natural process that seeks to reduce the binding energy in metals. The end result of corrosion involves a metal atom M being oxidized, whereby it loses one or more electrons and leaves the bulk metal,
M  Mm+ + m e- Mm+ + m e-The lost electrons are conducted through the bulk metal to another site where they reduce (i.e. combine with) a non-metallic element N or another metallic ion G+ that is in contact with the bulk metal,
N + n e-  Nn- Nn-
Gm+ + m e-  G GIn corrosion parlance, the site where metal atoms lose electrons is called the anode, and the site where electrons are transfered to the reducing species is called the cathode. These sites can be located close to each other on the metal's surface, or far apart depending on the circumstances. Anode/cathode pairs, known as corrosion cells, come in a variety of forms including composition cells (also known as Galvanic Cells), stress cells, and concentration cells. |
| Electrolytes and the Corrosion Circuit |
 |
| Corrosion is essentially an electric circuit, since there is a flow of current between the cathode and anode sites. In order for a current to flow, Kirchoff's circuit laws require that a circuit be closed and that there exists a driving potential (or voltage). Part of the corrosion circuit is the base metal itself; the rest of the circuit exists in an external conductive solution (i.e. an electrolyte) that must be in contact with the metal. This electrolyte serves to take away the oxidized metal ions from the anode and provide reduction species (either nonmetalic atoms or metallic ions) to the cathode. Both the cathode and anode sites must be immersed in the same electrolyte for the corrosion circuit to be complete. The most common electrolyte associated with corrosion is ordinary water. What provides the potential that drives the corrosion circuit? In most cases, the differences in the atom binding energies within a metal provide the driving potential (e.g. composition cells, stress cells). Ion concentration gradients in the electrolyte can also provide a potential (concentration cells). Note that inside the metal, the charge carriers are electrons; outside the metal, the charge carriers are ions dissolved in the electrolyte. |


 be an integer with representation
be an integer with representation  then n is a narcisstic number. For example, the decimal (
then n is a narcisstic number. For example, the decimal (
 then n is called a perfect digital invariant or PDI.
then n is called a perfect digital invariant or PDI.


 for some m.
for some m.





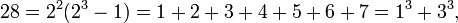
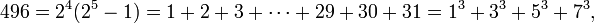
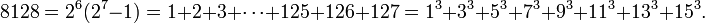

 > 1020 for some j (Cohen 1987).
> 1020 for some j (Cohen 1987). (Nielsen 2003).
(Nielsen 2003).
